LAPSES IN ALERTNESS: COHERENCE OF FLUCTUATIONS IN PERFORMANCE AND EEG SPECTRUM
Cognitive Performance and Psychophysiology Department,
Naval Health Research Center, San Diego, California
Current Address: Swartz Center for Computational Neuroscience, UCSD
Address correspondence to:
Keywords: EEG spectrum, coherence, sleep, alertness, vigilance, sonar
PDF download (.pdf.gz, 15.3MB)
Abstract
Thirteen subjects detected noise burst targets presented in in a white noise background at a mean rate of 10 per minute. Within each session, local error rate, defined as the fraction of targets detected in a 33-second moving window, fluctuated widely. Mean coherence between slow mean variations in EEG power and in local error rate was computed for each EEG frequency and performance cycle length, and was shown by a Monte Carlo procedure to be significant for many EEG frequencies and performance cycle lengths, particularly in four well-defined EEG frequency bands, near 3, 10, 13, and 19 Hz, and at higher frequencies, in two cycle length ranges, one longer than 4 minutes and the other near 90 s per cycle. The coherence phase plane contained a prominent phase reversal near 6 Hz. Sorting individual spectra by local error rate confirmed the close relation between performance and EEG power and its relative within-subject stability. These results show that attempts to maintain alertness in an auditory detection task result in concurrent minute and multi-minute scale fluctuations in performance and the EEG power spectrum.
Introduction
Methods and Materials
Results
Discussion
Conclusions
Acknowledgements
References
Since the landmark investigations of Mackworth (1948),
studies of alertness have confirmed that,
despite sincere intentions, few watchstanders
remain "unwaveringly" vigilant while
engaged in monotonous monitoring tasks.
Detection rates in laboratory tests
begin to degrade after only 2-3 minutes,
eventually reaching a plateau during which,
on average, only 70-80% of targets are detected
(Davies and Parasuraman, 1982).
Most vigilance research has only studied
mean trends in performance across subjects,
and ignored dynamics of performance within individual sessions.
However, there is evidence that
performance on target detection and other tasks
actually tends to fluctuate
with cycle lengths ranging from a few seconds to several minutes
(Seashore and Kent, 1905; Wertheimer, 1953;
Stroud, 1966; Stebel and Sinz, 1971; Warner, 1979;
Treisman, 1984; Makeig, 1985).
Unfortunately,
most vigilance experiments designed to simulate actual work environments
have used target presentation rates too low
to observe minute-scale performance dynamics.
Profound changes in the appearance and spectrum of the EEG
with sleep and drowsiness
have been noted since the first investigations of Loomis et al. (1937),
and EEG power band measures have been shown to correlate with
visually defined sleep stages (Rechtschaffen and Kales, 1968).
Matousek and Petersen (1983),
using a linear combination of over thirty
band and band-ratio amplitude measures,
were able to reproduce the classification of EEG epochs into awake
and Stage I sleep states by visual inspection,
and Penzel and Petzold (1989) observed that
hand-scored EEG vigilance estimates of sleep onset periods
could be predicted reliably
using measures of the cumulative power spectral distribution.
The link between EEG and arousal is well-enough accepted
that many studies of vigilance simply define vigilance
using electroencephalographic (EEG) and electrooculographic (EOG) criteria.
However, detailed studies of the appearance of the EEG
in drowsiness have noted that in different subjects
the EEG can take a variety of routes from wakefulness to sleep
(Santamaria and Chiappa, 1987).
Most studies correlating task performance and the EEG spectrum
have computed measures on one or more EEG spectral bands defined a priori
(Williams et al., 1962),
rather than computing full-spectrum correlations between
performance and behavior.
These reports have generally found that the correlation between EEG band
amplitudes and performance varies depending on performance measure, task,
subject state, and electrode site.
For example, Beatty et al. (1974)
reported that periods of lowered performance on a visual task
were predicted by lowered electrocortical
activation as indexed by a simple measure of
occipital theta band EEG amplitude,
and Townsend and Johnson (1979) showed that errors of omission in sleep-deprived
subjects were correlated with changes in the EEG spectrum,
although the structure of this relationship varied across subjects.
In our experiments, we present
simulated passive sonar targets
at a more rapid rate (10 per min.)
than has been used in most vigilance experiments.
We are then able to define a measure of performance, local error rate,
the fraction of targets detected in a moving time window,
and use it to study the electrophysiological correlates of changes
in performance.
We examine the relationship of minute-scale and multi-minute-scale
fluctuations in performance to concurrent changes
in EEG spectrum using three methods of analysis--
coherence, correlation, and error-rate sorted spectra.
The purpose of the experiments is twofold: first, to better
understand the dynamics and electrophysiological correlates
of lapses in detection performance,
and second, to assess the information available in the EEG that
might be used to perform automatic monitoring of operator readiness
to detect and respond to auditory signals.
Stimuli
Sound synthesis and data collection were controlled by a
Concurrent Real-Time Unix 68030 computer system
using 12-bit A/D and D/A converters
sampling at 5 and 50 kHz respectively.
Throughout each session, target and probe auditory stimuli
were presented binaurally through headphones mounted in
isolating cuffs in the presence of
continuous white noise background at 62 dB nHL.
Task-irrelevant auditory probe tones of 568 and 1098 Hz
were presented in random order at 72 dB nHL
with stimulus onset asynchronies (SOAs) between 2-4 s.
The high tone was presented more frequently (80%) than the low tone (20%).
Probe tones were 50 ms in duration with rise and fall times of 10 ms.
Evoked responses to the probe tones in these experiments
have been discussed elsewhere
(Makeig et al., 1990).
Target noise bursts were 300 ms in duration
with long rise and fall times of 150 and 110 ms respectively.
Targets occurred pseudorandomly in 50% of the inter-probe intervals,
giving a mean target rate of 10 per minute.
Temporal positions of the target onsets were also pseudorandom,
but did not occur within 400 ms of the nearest probe tone.
Target intensity was set at 6 dB above its relative threshold in the noise
as determined by pre-testing on 10 normal-hearing laboratory workers.
This intensity was high enough to allow near-perfect initial performance levels,
but low enough not to startle subjects nor delay onset of decrements in alertness.
Steady-state click probe stimuli were also presented continuously
throughout the experiment at a rate of 39.0625 Hz
(an exact sub-multiple of the EEG and sound sampling rates)
and an intensity of 63 dB nHL.
Against the noise background, the click train was perceptible but not intrusive.
The purpose of this stimulation was to evoke
an auditory steady-state response (SSR) at the stimulus rate
(Galambos et al., 1981; Makeig, 1985).
Subjects
A total of thirteen males participated
in a simulation of a passive sonar auditory target detection task.
Of these, nine were prospective students in the Navy sonar course,
three were sonar instructors, and one was from the laboratory staff.
Ages ranged from 18 to 34 years (mean 24).
All had passed standard Navy hearing tests.
Each subject participated in two simulated work sessions of 28 minutes.
Procedure
.
Subjects sat in a comfortable chair with eyes closed,
their right index finger resting on a response button.
They were instructed to press the button as soon as possible
each time they detected a target noise burst, and to ignore the probe tones.
The instruction to keep eyes closed was used to increase the difficulty
of remaining alert throughout the session.
Data collection
Data from all sessions were continuously recorded to disk for off-line analysis.
EEG and EOG signals were amplified 50K times with a 0.1-100 Hz bandwidth
through Grass EEG amplifiers, multiplexed with button press information
and converted to 12-bit digital format at a sampling rate of
312.5 Hz per channel
(exactly 8 times the steady-state probe repetition rate).
EEG was collected from 13 scalp locations
of the International 10-20 system (for locations see Fig. 5).
An ECI Electro-Cap provided standardized placement of Ag/AgCl electrodes,
all referred to the right mastoid.
Electrical impedances at all electrode sites were less than five kOhms.
Periocular electrodes were used to record electrical potentials generated by
movements of the eyes during EEG recording.
and to reject from analysis data epochs containing large eye movements.
Local error rate
To test for linear relationships between
alertness and the EEG power
spectrum, a continuous estimate of performance at regularly
spaced time intervals was necessary.
Such a measure, the local error rate, was derived by computing
the fraction of undetected targets within a time window with a constant width of
32.8 s which was advanced through the data in steps of 1.64 s.
Targets were considered detected if the subject pressed the response button
within a relatively wide time window of 200-4000 ms following stimulus onset.
For the few instances in which no targets occurred
within an error rate window,
the value of the previous overlapping window was assigned to it.
Averaged across all 26 sessions, the mean error rate followed the
pattern typical in vigilance studies and the
local error rate covaried linearly with mean reaction time
(Makeig et al., 1990).
If the targets had been presented at evenly-spaced intervals,
computation of local error rate
from the raw performance data would amount to
low-pass filtering the performance data with a moving-average filter,
a linear operation.
But since the targets occurred at semi-random intervals,
the local error rate was derived nonlinearly
and had spectral contents not found in the raw data.
To assess the significance of
the deviation of observed fluctuations from expected values,
we compared the mean of the actual error rate spectra
with a Monte Carlo distribution
of spectra of synthesized local error rate time series
derived from the real data by "shuffling" it,
i.e. retaining the same target presentation times and performance level,
but randomly permuting the order of the detected and undetected targets
and then applying the same error-rate algorithm as was applied to the
actual data.
This procedure was carried out 200 times
and the results were averaged to determine the spectrum expected had
the same responses and lapses been randomly ordered.
Coherence
To detect linear relationships between
fluctuations in performance and changes in the EEG power spectrum,
we estimated the coherence
between the local error rate series
and the corresponding EEG power spectrum series.
Note that this coherence measure is very different from measures of
coherence between EEG waveforms at different scalp sites
(Rappelsberger and Petsche, 1988).
Here, coherence is used to measure the degree to which
slow changes in EEG power at one site accompany slow changes in
local error rate, a heuristic estimate
of the current probability that a target will be detected.
Our plots below show the square root of coherence amplitude
or coherency (Brillinger, 1981), and coherence phase.
In our computations,
each error rate time series consisted of 1024 points at 1.64 s intervals.
The EEG power spectrum time series consisted of 1024 power estimates
for each of 81 EEG frequencies
spaced at 0.61 Hz intervals, from 0.61 to 49.41 Hz
(note-3).
Significance Level
A nonparametric Monte Carlo procedure was used to assess
the significance of the results.
Statistical theory exists for assessing significance of coherency
for Gaussian time series (Brillinger,1981),
but due to the frequent saturation of the
local error rate series at 0.0 and 1.0 values and resulting non-normality,
we instead determined the expected and critical values
for coherency
by estimating its distribution under the null hypothesis.
To simulate statistical independence
and destroy any expected linear relationship
between local error rate and EEG power
without altering their amplitude spectra,
we randomly permuted the order of the error rate FFT's
and computed the coherency of the mismatched pairs.
This process was repeated 200 times and results averaged
to generate expected coherency distributions
(note-4).
From these the mean, standard deviation, skew, and kurtosis
of coherency under the null hypothesis
were computed for each cycle length and EEG frequency.
Using these four statistics and the percentile function
outlined in Ramberg et al. (1979),
we then estimated the p=0.01 critical values
for coherency at each cycle length and EEG frequency.
Local Error Rate
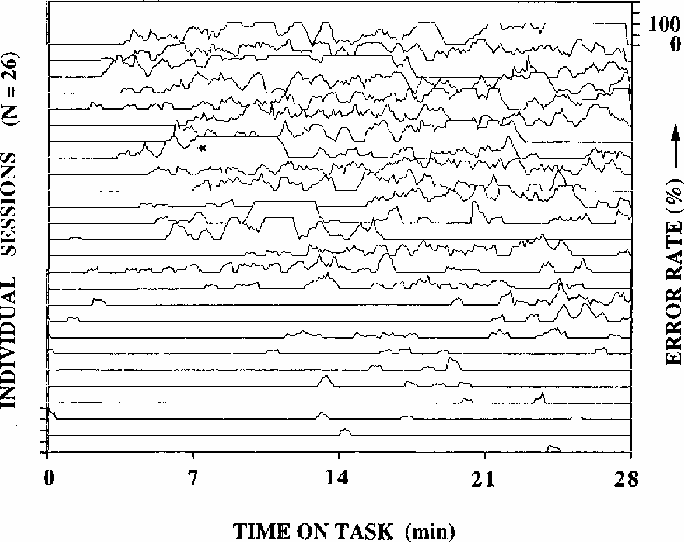
Figure 1
Local error rates during 26 half-hour test sessions
(2 sessions from each of 13 subjects).
Adjacent traces are not necessarily from the same subject; rather,
sessions are arranged from top to bottom of the figure by decreasing
mean error-rate. Error rates are computed using a moving 33-second wide
window. Note the wide fluctuations in error rate, including the
complete absence of responding for several minutes
in the run marked with an asterisk. After this session,
the subject did not recall the episode.
Figure 1
Local Error Rate Spectrum
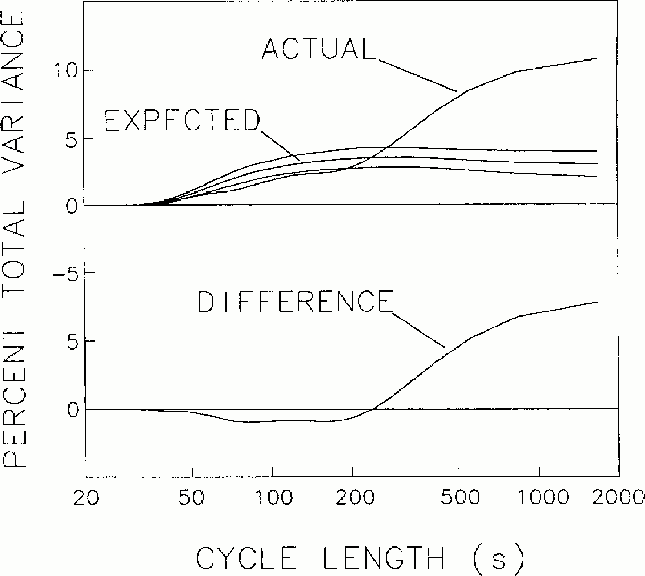
Figure 2
Mean spectrum of fluctuations in local error-rate, with the spectrum
expected from Monte Carlo simulation and 2 s.d. bands. Lower trace.
The difference between the actual and expected error rate spectra, with
larger values than by chance occurring
at cycle lengths longer than about 4 minutes per cycle.
Figure 2
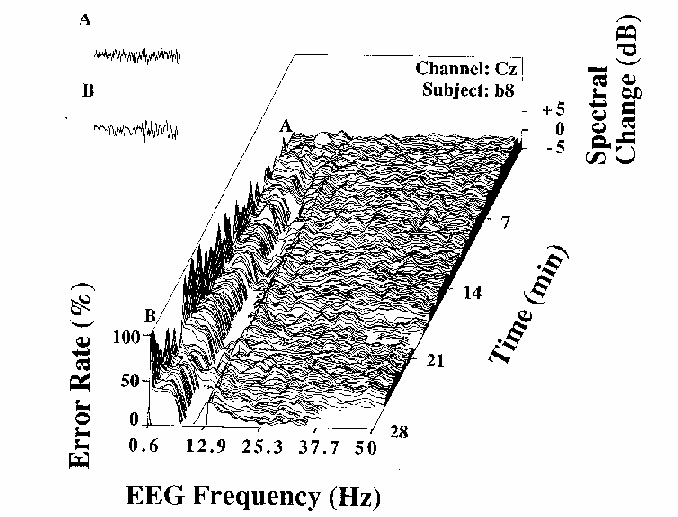
Figure 3
Error rate and EEG spectral power
(normalized by converting to dB
and subtracting the mean log spectrum for the first two minutes)
during a 28 minute session for one subject (b8).
The gradually increasing fluctuations in local error-rate
are plotted to the left of the spectral plane.
Note the relationship between changes in the EEG spectrum
and changes in local error-rate.
These are illustrated by two 4 sec EEG segments (upper left)
taken from moments in the session marked A and B in the figure.
These excerpts demonstrate a contrast
between dominant near-10 Hz activity when error rate is low (A),
and near-4 Hz activity when error rate is high (B).
Figure 3
Coherence
The sample coherence between local error rate and time-varying power in each
of 81 different EEG frequencies was computed across thirteen sessions
from ten subjects for a range of 36 cycle lengths. Results are depicted
for site Cz in Figure 4
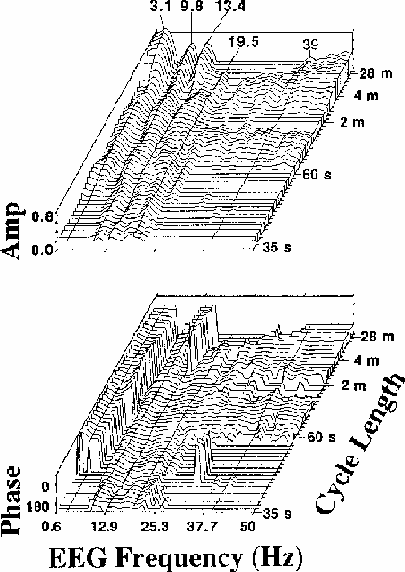
Figure 4.
Coherency (a) amplitude and (b) phase planes for channel Cz
(averaged over
the same 13 sessions as Fig. 2)
for a wide range of EEG frequencies (0.6-50 Hz)
and error rate fluctuation cycle lengths (35 s to 28 m).
Only those (frequency x cycle length)
combinations having significant coherency
(p < .01 by Monte Carlo simulation) are plotted above baseline;
insignificant data points are plotted using baseline values.
EEG frequency sampling width is 0.61 Hz.
Data at the five EEG frequencies with highest coherence
(3.4, 9.8, 13.4, 19.5 and 39 Hz) are marked.
Note the significant coherence of alpha and beta-range EEG (10-20 Hz)
with error rate at all cycle lengths,
and the sharp 180 degree reversal in coherence phase near 6 Hz,
running along the left side of the coherency phase plane (b)
which plots coherency (square root of coherence amplitude) and phase
for all points at which the coherency
value exceeds the p = 0.01 critical value
determined by the Monte Carlo procedure
(note-5).
Locations at which coherency is below the critical value
are indicated by baseline coherency and coherence phase planes
(plotted as critical coherency and -270 degrees phase respectively).
Several features of these coherence planes merit comment:
There is strong evidence for a linear relationship
between the fluctuations in EEG power and performance
at all cycle lengths and almost all EEG frequencies.
Three relatively narrow EEG spectral bands,
with maxima near to 3, 10, and 13 Hz,
dominate the coherency plane at Cz.
At the longest cycle lengths,
coherency is non-significant
in two narrow EEG bands near 7 and 12 Hz,
the null near 7 Hz extending across all cycle lengths.
The corresponding phase plane shows that
this coherency trough accompanies a reversal
in coherence phase from near 0 to 180 degrees (left side of Fig. 4b).
That is, fluctuations in EEG power below 6 Hz are in phase
(and positively correlated) with error rate fluctuations,
while fluctuations above 7 Hz are near 180 degrees out of phase
(and negatively correlated) with error rate fluctuations.
At almost all EEG frequencies,
the flatness of the coherence phase plane
with respect to cycle length indicates
there is no appreciable time lag between
changes in error rate and EEG power.
That is, changes in performance tend to be accompanied by
nearly simultaneous shifts in EEG spectral power
(note-6).
A second region in coherence phase
occurs for EEG frequencies near 13 Hz,
where, at longer error rate cycle lengths,
rise in error rate is
accompanied by an increase in 13 Hz EEG spectral power.
However, for these same frequencies at shorter cycle lengths
(as for all other EEG frequencies above 7 Hz),
rise in spectral power is correlated with a decrease in local error rate.
Note also that between 10 and 20 Hz,
significant coherence extends to the
shortest cycle length shown (35 s), indicating that power
at these EEG frequencies reliably tracks even comparatively rapid
error rate fluctuations
(note-7).
Details of the coherency plane
suggest that fluctuations in error rate
with different cycle lengths may have different EEG concomitants.
As cycle length decreases from 28 minutes
to near one minute per cycle,
the locus of maximum coherency
appears to seesaw above and below an EEG frequency fulcrum at about 20 Hz.
At the longest cycle lengths, EEG frequencies below 15 Hz
dominate the coherency plane,
while in the range 15-39 Hz, coherency is insignificant.
At 3-4 min per cycle, at high EEG frequencies coherency are maximum,
while at low frequencies, coherency reaches relative minima.
Near two minutes per cycle, there are local peaks in the three
low-frequency EEG bands and a null in coherency above 20 Hz,
while below 60 s per cycle, for high EEG frequencies,
coherency again becomes insignificant,
but modest peaks are present in the 3 and 10 Hz bands.
While the significance of these cycle length regions is not known,
they appear to be distinguishable phenomena,
since time scale frequency structure is not visible in
the Monte Carlo coherency plane simulations.
Finally, the small peak
at the steady-state response rate, 39 Hz,
in the coherency plane (Fig. 4a)
implies that some portion of the EEG at 39 Hz in this experiment,
most likely the auditory SSR driven in these experiments
by the 39 Hz click train,
is independently correlated with fluctuations in performance.
This may be due to the influence of central brain systems
modulating the output
(Galambos and Makeig, 1988; Steriade and Biesold, 1990)
of the primary generator of the SSR in the auditory cortex
(Romani et al., 1983; Makela and Hari, 1987).
Scalp Topography
In these experiments, significant coherency
between EEG and performance was not confined to a single scalp site.
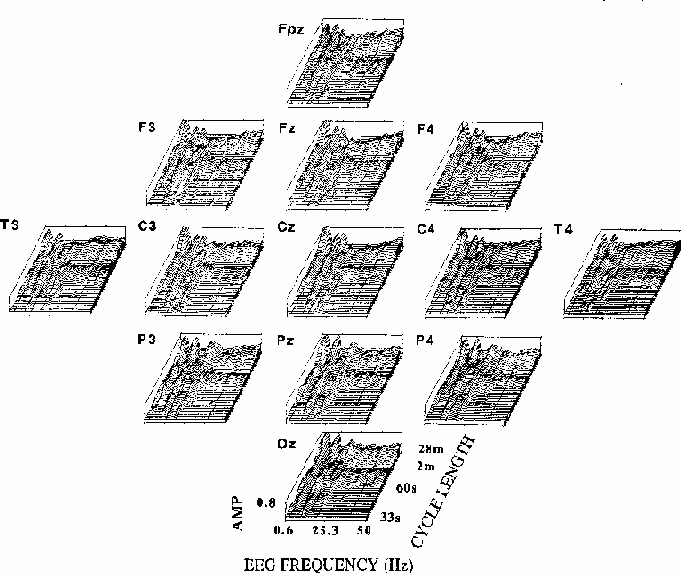
Figure 5
Figure 5.
Coherency amplitude planes (computed as in Fig. 4) for all 13 scalp sites.
Note the similarity of EEG frequency and cycle length bands across channels,
and between-site differences including the prominent coherency near 19 Hz
at occipito-parietal sites, near 13 Hz at fronto-central channels,
and the relative maximum at fronto-central sites for long cycle lengths
at 39 Hz, the steady-state driving frequency.
The overall shape and extent of regions of significant coherency
are similar at all scalp locations,
but close inspection reveals between-site differences;
note the 13 Hz peak in coherency for long cycle lengths
at central sites, and the 19 Hz peak at posterior sites.
The relatively small but well-defined coherency peak
at 39 Hz seen most clearly at site Fz
is largest at fronto-central sites, where the SSR is also largest
(Galambos et al., 1981),
and coherency at 10 Hz appears stronger at the right temporal (T4)
than at the left temporal (T3) site.
Note also that the zone of significant coherency near 90 s per cycle
is present at all sites for EEG frequencies above 25 Hz.
Correlation
Since coherence analysis reveals no significant lags between
performance and EEG, correlation can also be used to measure
the linear relation between the two time series.
Since most spectral variance in the error rate time series occurred for
cycle lengths greater than 4 minutes,
the EEG and error rate series were smoothed using a 2 minute moving-average
filter to eliminate variance at cycle lengths shorter than about 3 minutes.
Then for each session, site, and EEG frequency,
correlations between the EEG power and local error rate
time series were computed separately.
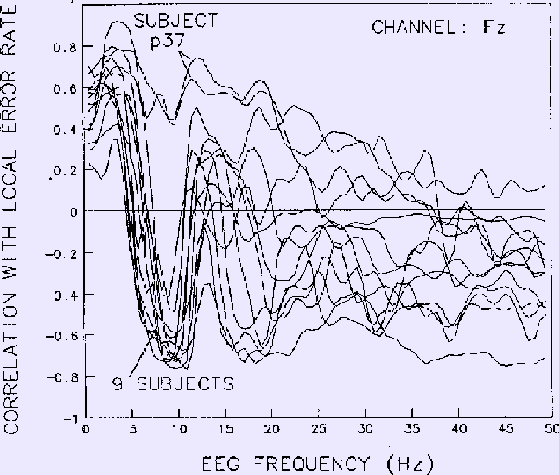
Results for each frequency at the site (Fz) yielding strongest correlations
are shown in Figure 6
Figure 6. Correlations between EEG power at Fz and local error rate
for each EEG frequency.
Same 13 sessions on 10 subjects as in Figs. 4-5.
Data smoothed using 2-minute moving windows.
Note the strong negative correlation near
10 Hz for all sessions except those of subject p37, whose EEG
spectrum did not contain an alpha band peak.
Note also the net zero correlation in the 5-7 Hz region, in accord with
the phase reversal near 6 Hz in the coherency phase plot (Fig. 4b).
At frequencies lower than 16 Hz, results for nine of the
ten subjects are highly similar.
Two similar results from one subject (p37), however,
have no negative correlation peak near 10 Hz.
Examination of individual subject EEG spectra confirmed that
unlike the other nine subjects,
this subject's EEG spectrum contains no peak in the alpha frequency range.
At EEG frequencies above 20 Hz in Fig. 6,
mean correlation between EEG power and performance is nearly flat,
although between-subject variability is largest in this range.
The phase reversals seen in the Cz coherence phase plane (Fig. 4b)
are also seen in these Fz spectral correlations.
In particular, the strong reversal in coherence phase at about 6 Hz is
paralleled by reversal of the correlation sign
for all nine subjects with alpha peaks in their EEG spectra.
As with coherence,
mean correlation with performance is largest near 3 and 10 Hz.
Error-Rate Sorted Spectra
As a means to confirm our coherence results directly, and to investigate
the relationship between performance and EEG within single sessions,
we measured the change in the EEG power spectrum
as a function of local error rate
by sorting the power spectra from each session according to
concurrent local error rate,
then performing a moving average using a constant width (30%)
error rate window
which was advanced through the sorted spectra
in small (2%) steps.
To reveal spectral changes more clearly, we normalized the
error-rate sorted spectra
by dividing each spectrum by the zero error rate spectrum,
i.e. by the average power spectrum of all 2.46 s epochs centered in
at least 33 s of error-free performance.
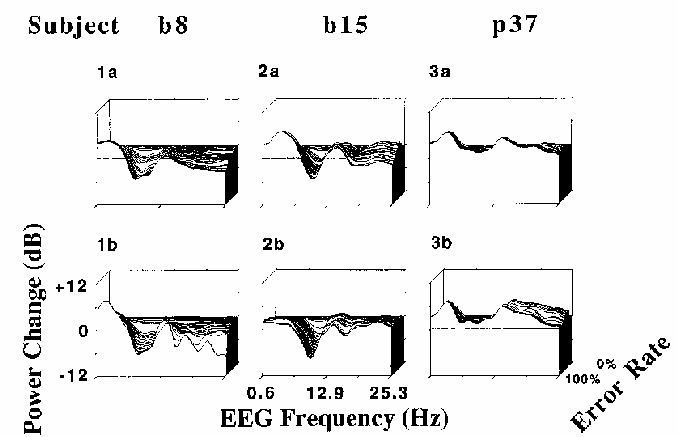
Figure 7
Figure 7
Error-sorted EEG spectra,
normalized by dividing by the mean zero error-rate
spectrum, sorted by local error rate, and then averaged using a moving window
of constant (30%) error-rate width and (2%) step-size.
Results of two sessions each are shown for three subjects
(p37, b8, and b15).
Note the within-subject similarities in the spectral changes
accompanying increasing local error rate,
as well as the between-subject differences.
In Fig. 7, depth indexes local error rate --
greater depths corresponding to lower error rates.
In each plot, the deepest (and highest) trace
represents the mean EEG spectrum of the
0% error rate epochs, normalized to unity.
The frontmost trace represents the ratio between the mean of the
100% error rate epoch spectra and the 0% error rate mean, log scaled in dB.
The horizontal axis indexes EEG frequency from 0.6 to 25 Hz.
The results of the error-rate sorted spectra were consistent with
results of the coherence and correlation analyses.
As local error rate increased, so did power in frequencies below 7 Hz,
while above 7 Hz (and particularly near 10 Hz for most subjects),
increases in error rate corresponded in general to decreases in EEG power.
Note that the location of peaks in the error rate spectra
in Fig. 7 appear to be identical between sessions,
although they differ between subjects.
The locations of peaks and troughs in the error-sorted spectra are correlated
with locations of peaks in same subjects' mean waking EEG spectra (not shown)--
subject b8 has a prominent peak at 8 Hz, while subject b15
has a clear peak near 10 Hz, and subject p37 has no alpha range peak
at all.
To evaluate
the nature and strength of the linear relationship
between alertness and the EEG spectrum,
we performed a coherence analysis of fluctuations in moving-average measures
of performance and EEG power.
Results of the coherence analysis were verified using
correlation and error-sorted spectral averaging.
These analyses provide strong converging evidence that
changes in performance on this auditory detection task
are linearly related
to specific patterns of changes in the EEG power spectrum
at all EEG recording sites and over a wide range of performance cycle lengths,
and that this relationship is relatively variable across but stable within subjects.
EEG Band Structure
The long-time mean EEG spectrum
has a predominant smooth character (Nunez, 1981),
with little band structure evident
other than, for most subjects, a dominant peak in the alpha range.
A striking result of our coherence analysis is the sharp,
behaviorally defined frequency band structure it reveals.
For the most part, the stable band structure uncovered by this analysis
conforms to traditional EEG bands concepts; strong peaks in coherence
appear at delta, alpha, sigma, and beta bands, and these have distinct
scalp topographies. However, the critical crossover frequency
between cophasic and antiphasic coherence occurs near 6 Hz,
the center of the traditionally defined (5-7 Hz) theta band.
The analysis, therefore,
also reveals the limitations of using a priori frequency band averaging
to study changes in the EEG spectrum.
For 9 of the 10 subjects analyzed, plots of correlation between EEG power
and behavior have at least two features in common --
EEG power below 6-7 Hz is highly positively correlated,
and EEG power near 10 Hz highly negatively correlated with local error rate --
these correlations approaching unity
for fluctuations slower than 3 minutes per cycle.
The 13 Hz in-phase peak at long cycle lengths in the coherence planes (Fig. 4)
may reflect the emergence of
sleep spindles during protracted periods of high error rates.
However, Roschke and Aldenhoff (1991) have also noted the emergence of
a peak near 13 Hz
in the spectrum of auditory and visual evoked responses during sleep.
It is also possible, therefore, that the 13 Hz coherency peak in Fig. 4
represents EEG activity
prior to emergence of visible sleep spindling
or its precursor (Hari, 1985).
Our coherence results appear to contradict the claim of Beatty et al. (1974)
that occipital theta band EEG is the most reliable spectral indicator
of vigilance.
Nor do they confirm the recent claim of Ogilvie et al. (1991)
that power in all bands increases immediately preceding errors of
omission.
(note-8).
However, differences in task (our subjects
were asked to attempt to continue performing the task
throughout the session)
and conditions (our subjects' eyes were closed) could account
for some differences from other experiments.
Most previous studies of EEG and vigilance have used relatively low performance
sampling rates which could not be used to reveal the time-structure
of fluctuations in performance and EEG or their interrelationship.
In this study,
analysis of coherence between EEG and performance appear to reveal a band
structure in performance cycle lengths
orthogonal to the frequency band structure of the EEG itself.
We do not have an explanation for the distinct band of
coherence at cycle lengths near 90 s which
appears at higher EEG frequencies in the coherency plane.
This feature reflects an apparent tendency for
behavior and high frequency EEG power to covary on this time scale,
although it is not accompanied by peaks in
the raw error rate spectrum (Fig. 2).
However, although there has been relatively little study
of basic neurophysiological processes
that may underly minute-scale performance fluctuations
(Churchland and Sejnowski, 1988),
fluctuations on the 100 second scale
have been reported
for memory and magnitude estimation tasks
(Wertheimer, 1953; Augenstein, 1955; Stebel and Sinz, 1971;
Treisman, 1984),
and, in subcortical multi-unit activity,
as increasing in strength during drowsiness
(Moiseeva and Aleksanian, 1986).
It is possible, therefore, that the 90 s EEG coherence phenomenon
might have more general behavioral correlates.
When we feel drowsy, we often say we feel "half-awake."
The smoothed measure of performance
we construct here and then predict from fluctuations in EEG spectral power,
local error rate, gives a behavioral index
of alertness or wakefulness also ranging between zero to one.
Technically, however, our local error rate measure is the result
of lowpass filtering an alternating series of hits and lapses.
For example, a local error rate of 0.5 means
that of the approximately 6 targets presented in a 33 s
moving window, 3 were detected and 3 not detected.
It is possible that rather than remaining in a 50%-alert
state throughout the 33 s window, more rapid fluctuations in
both alertness and electrophysiology are actually taking place
which our local error rate does not measure,
perhaps connected to
the reported 10-20 s cycles in
alpha abundance
(Evans, 1991) and
multi-unit firing rates
(Moiseeva and Aleksanian, 1986),
or the near 40 s cycles reported in sleeping EEG
(Scheuler et al., 1987;
Terzano and Parrino, 1988;
Pastelak-Price et al., 1990)
Although coherence analysis
can detect phase shifts other than 0 or 180 degrees
and time leads or lags of up to a half a performance cycle,
our coherence results give no evidence for either.
That is, the linear relation between EEG and vigilance in these experiments
did not involve a measurable constant time lag between one and the other.
This is not to say, however, that higher-order, non-linear measures
may not reveal time ordering of changes in EEG and behavior. For example,
Hori (1985) has claimed that increased variance, a second-order measure,
of sigma (13 Hz) EEG power
precedes the onset of decrement in alpha power at transition to sleep.
Elsewhere we have reported the relationship between local error
rate and averaged brain responses evoked by the task-irrelevant
probe tones presented during these experiments (Makeig et al.,
1990), and discussed the prospects of using evoked responses to
estimate performance. However, for such purposes evoked responses
have two drawbacks: they can only be recorded intermittently, and
they must then be averaged or otherwise aggregated to separate them
from the EEG background, which is itself continuously changing with
subject state. EEG monitoring, on the other hand, can be continuous,
and power of the ongoing EEG is nearly always greater than power
in evoked responses.
Since strong linear relationships between
EEG spectral power and local error rate are indicated by the
coherence and correlation analyses, a lower bound on the performance
of an optimum algorithm for estimating vigilance level from EEG
data can be obtained from multiple linear regression. To demonstrate
a potential application of our results, the clear band structure
visible in results of the coherence analysis was used to select
five characteristic frequencies to enter into a multiple regression
predicting local error rate from EEG power. These EEG frequencies
are those traced in Figs. 4 and 5. For each recording site, power
at these five frequencies and the local error rate time series were
each normalized to zero mean and unit variance and entered into a
standard multiple regression algorithm
(note-9).
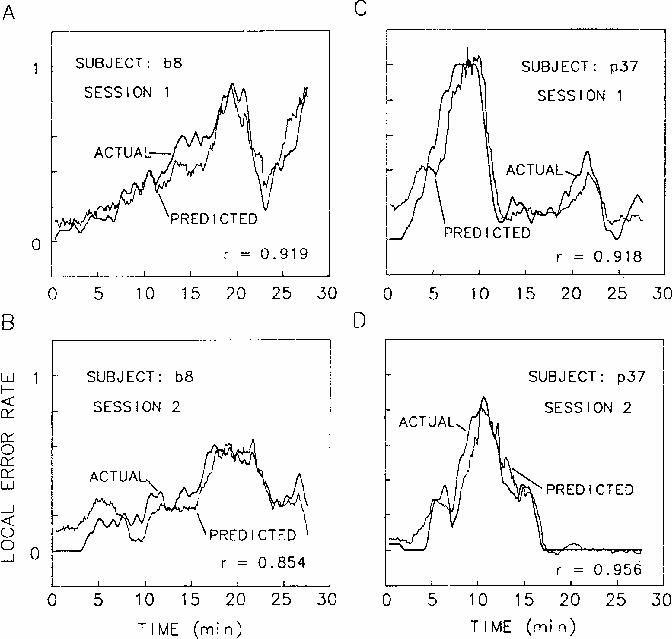
The upper panels of Figure 8
plot the local error rate time series
superimposed on the best fitting linear regression estimates
of local error rate,
from EEG power at site Cz,
Figure 8.
Linear regressions of EEG power at Cz on error rate using the 5 coherence
peak frequencies marked on the coherence planes (Fig. 4),
overplotted against actual error rate records.
Two-minute moving windows were used
to average both performance and EEG data.
For two subjects (b8 and p37), EEG from
sessions shown in the upper panels
was used to determine regression weights.
Success in predicting local error rates
using these same weights
on separate sessions from these same two subjects
for first sessions of two subjects.
Since coherence analyses indicated maximum coherence
computed from EEG power at each site occurs for cycle lengths
of four minutes and longer,
we computed the multiple regressions using
error rate and power spectrum data
using a two-minute moving window.
In the lower two panels,
the regression equations derived to fit the first sessions
were used to predict error rates in second sessions for the same subjects.
The subject in the right panels was the low-alpha subject, p37.
The subject shown on the left, b8,
had an alpha peak in his EEG spectrum,
and this difference was reflected
in the different relative weights of the two subjects'
3 and 10 Hz regression weights.
However,
as can be seen in the figure,
for both subjects the regression procedure
accurately predicted the changes in
local error rate that occurred throughout the sessions.
Note that these results do not
imply that all types of errors of omission
are predictable from mean changes in the EEG spectrum.
In particular, isolated errors during periods of low error rate
may not be detectable,
even though the linear regression prediction error
may remain low (see for example Fig. 8d, end of session).
Some performance lapses might arise from
momentary distraction, failure of signal detection, willful neglect, etc.,
factors not necessarily accompanied by changes in arousal or mean EEG spectrum.
As Townsend and Johnson (1979) state,
"changes in the frequency content of the EEG appear to be
predictive of performance only when they are secondary to changes
in arousal which in turn affects performance."
However, the high coherency values obtained in these experiments
suggest that under laboratory conditions,
most detection errors can be accounted for by the
existence of multi-minute scale fluctuations in alertness
associated with slow changes in the EEG power spectrum.
It is possible that fluctuations in performance
on more cognitively demanding tasks might be better predicted
by somewhat different combinations of EEG spectral information
(Belyavin and Wright, 1987).
In our experiments,
the strength and character
of the relationship between performance and alpha band EEG
may have been partly determined by our instruction to subjects
to keep their eyes closed during these session.
Under eyes open conditions, alpha EEG power has been observed
to first increase and then decrease with increasing drowsiness
(Torsvall and Akerstedt, 1988), a relationship which may complicate
alertness estimation.
Future work should explore the relation
of fluctuations in EEG and performance
of complex cognitive tasks with eyes open,
both features of most work environments in which alertness monitoring
might find practical use.
Our results show that
changes in EEG power constantly accompany
slow and irregular, multi-minute and near-minute scale fluctuations
in arousal and cognitive state of which we may not be fully aware.
The changes in the EEG spectrum accompanying performance changes
in these experiments appear to be those associated with drowsiness,
though, like the EEG spectrum itself,
the exact pattern of correspondence between EEG and behavior
differs for different individuals.
This relationship
appears to remain stable within individuals across sessions,
a fact which could lead to practical applications
involving alertness monitoring.
The authors wish to thank Steven Hillyard, Terrence Sejnowski,
Laverne Johnson, and Robert Galambos
for helpful advice and criticism of early versions of this paper,
and to acknowledge the valuable contributions of F. Scot Elliott in
collecting and archiving the data, and of Lcdr. David Kobus
for his enthusiastic support of the project.
Portions of this data were presented at meetings of the Human Factors Society,
Orlando, 1990, the Society for Psychophysiological
Research, Chicago, 1991, and the Society for Neuroscience,
New Orleans, 1991.
Augenstein, L.G. Evidences of periodicities in human task
performance. In: Quastler (Ed.), Information Theory in
Psychology: Problems and Methods. The Free Press, Glencoe, Ill, 1955
Beatty, J., Greenberg, A., Deibler, W.P., and O'Hanlon, J.F.
Operant control of occipital theta rhythm affects performance in a
radar monitoring task. Science, 1974, 183:871-873.
Belyavin, A. and Wright, N.A. Changes in electrical activity of the brain with
vigilance.
Electroenceph. clin. Neurophysiol., 1987, 66:137-144.
Brillinger, D.R. Time Series: Data Analysis and Theory.
Holden-Day, San Francisco, 1981.
Bendat, J.S. and Piersol, A.G.
Random Data: Analysis and Measurement Procedures,
second edition.
Wiley, New York, 1986.
Churchland, P. and Sejnowski, T. Perspectives on Cognitive Neuroscience.
Science, 1988, 241:741-745.
Davies, D.R. and Parasuraman, R.
The Psychology of Vigilance,
London: Academic Press, 1982
Evans, B.M. Endogenous rhythmic phasic activity in arousal mechanisms in man.
Proceedings of the Physiological Society, Sheffield meeting, 1991, 57P.
Galambos, R. and Makeig, S. Dynamic changes in
steady-state potentials.
In: E. Basar (Ed.), Dynamics of
Sensory and Cognitive Processing of the Brain,
Springer, Berlin, 1988, pp. 102-122.
Galambos, R., S. Makeig, and P. Talmachoff, A 40 Hz
auditory potential recorded from the human scalp, 1981,
Proc. Natl. Acad. Sci. USA, 78(4):2643-2647.
Hord, D.J. An EEG predictor of performance decrement in a vigilance task.
Technical Report 82-2, 1982, Naval Health Research Center, San Diego.
Hori, T.
Spatiotemporal changes of EEG activity during waking-sleeping transition period.
Intern. J. Neurosci., 1985, 27:101-114.
Johnson, L., Lubin, A., Naitoh, P., Nute, C., and Austin, M. Spectral analysis
of the EEG of dominant and non-dominant alpha subjects during waking and sleeping.
Electroenceph. clin. Neurophysiol., 1969, 26:361-370.
Loomis, A.L., Harvey, E., and Hobart, G.A. Cerebral states during human
sleep as studied by human brain potentials.
J. Exp. Psychol., 1937, 21:127-144.
Mackworth, N.H. The breakdown of vigilance during prolonged
visual search. Q. J. Exp. Psychol., 1948, 1:6-21.
Makeig, S., Studies in Musical Psychobiology.
University Microfilms, Ann Arbor, MI., 1985.
Makeig, S., Elliott, F.S., Inlow, M., and Kobus, D.A.
Predicting lapses in vigilance using brain evoked responses to
irrelevant auditory probes,
Technical Report 90-39, Naval Health Research Center,
San Diego, 1990.
Makela, J.P. and R. Hari, Evidence for cortical origin
of the 40 Hz auditory evoked response in man,
Electroenceph. clin. Neurophysiol., 1987, 66:539-546.
Matousek, M. and Petersen, I. A method for assessing alertness fluctuations
from EEG spectra. Electroenceph. clin. Neurophysiol., 1983, 55:108-113.
Moiseeva, N.I. and Aleksanian, Z.A.
Slow-wave oscillations of the multi-unit activity
average frequency in the human brain during drowsiness and sleep.
Electroenceph. clin. Neurophysiol., 1986, 63:431-437.
Nunez, P., Electric Fields of the Brain,
Oxford University Press, 1981.
Ogilvie, R.D., Simons, I.A., Kuderian, R.H., MacDonald, T., and Rustenburg, J.
Behavioral, event-related potential, and EEG/FFT changes at sleep onset.
Psychophysiology, 1991, 28:54-64.
Penzel, T. and Petzold, J.
A new method for the classification of subvigil stages,
using the Fourier transform, and its application to sleep apnea.
J. Comput. Biol. Med., 1989, 19:7-34.
Ramberg, J.S., Tadikamalla, P.R., Dudewicz, E.J., and Mykkytka, E.F.
A probability distribution and its uses in fitting data.
Technometrics, 1979, 21:201-214.
Rappelsberger, P., and Petsche, H. Probability mapping: power and coherence
analyses of cognitive processes. Brain Topography, 1988, 1:46-54.
Rechtschaffen, A., and Kales, A., eds. Manual of Standardized Terminology,
Techniques and Scoring System for Sleep Stages of Human Subjects.
Public Health Service, Bethesda, Maryland, 1968.
Romani, G.L., S.J. Williamson, and L. Kaufman, Characterization
of the human auditory cortex by the neuromagnetic method.
Exp. Brain Res., 1983, 47:381-393
Roschke, J. and Aldenhoff, J.B. Excitability and susceptibility of the brain's
electrical activity during sleep: an analysis of late components of
AEPs and VEPs.
Intern. J. Neuroscience, 1991, 56:255-272.
Scheuler, W., Rappelsberger, P., Schmatz, F.,
Pastelak-Price, C., Petsche, H. and Kubicki, S.
Periodicity analysis of sleep EEG in the second and minute ranges - example
of application in different alpha activities.
Electroenceph clin Neurophysiol, 1990, 76: 222-234.
Santamaria, J. and Chiappa, K.H. The EEG of drowsiness in normal adults.
J. Clin. Neurophysiol., 1987, 4:327-382.
Seashore, C.E. and Kent, G.H. Periodicity and progressive change
in continuous mental work. J. Psychol. Reviews, 1905, 6:47-101.
Stebel, J and Sinz, R.
On central nervous minute-periodicity and its coordination.
J. Interdisciplinary Cycle Res., 1971, 2:63-72.
Stroud, J.M. The fine structure of psychological time.
Ann. NY Acad. Sci., 1966, 138:623-631.
Terzano, M.G. and Parrino, L.
The cyclic alternating pattern sequences in the dynamic
organization of sleep.
Electroenceph. clin. Neurophysiol., 1988, 69:437-447.
Torsvall, L. and Akerstedt, T.
Extreme sleepiness: Quantification of EOG and EEG
parameters. Intern. J. Neurosci., 1988, 38:435-441.
Townsend, R.E. and Johnson, L.C.
Relation of frequency-analyzed EEG to monitoring behavior.
Electroenceph. clin. Neurophysiol., 1979, 47:272-279.
Treisman, M. Temporal rhythms and cerebral rhythms.
In: J. Gibbon and L. Allan (Eds.), Timing and Time
Perception. New York Academy of Sciences, 1984, 423:542-565.
Warner, R.M. Periodic rhythms in conversational speech.
Lang. Speech, 1979, 22:381-396.
Wertheimer, M. An investigation of the "randomness" of threshold measurements.
J. Exp. Psychol., 1953, 45:294-301.
Williams, H.L., Granda, A.M., Jones, R.C., Lubin, A., and Armington, J.C.
EEG frequency and finger pulse volume as predictors of reaction time during
sleep loss. Electroenceph. clin. Neurophysiol., 1962, 14:64-70.
3.
These were computed from a total of 2.46 s
of artifact-free EEG data by averaging the spectra from five 50%-overlapped,
0.82 s, Hanning-windowed data segments zero-padded to 512 points.
To derive the coherency estimate,
each 1024-point error rate and EEG power series was segmented into
thirteen 256-point blocks with 75% overlap.
These were then Hanning-windowed and zero-padded to 1024 points
and transformed to the frequency domain using FFTs.
Since each of the thirteen sessions contributed 13 FFT's,
the average coherency was computed from a total of 169 FFT's.
Return to text.
4.
Initially, two shuffling procedures were compared,
one in which the coherence
of shuffled error rate data was computed against randomly selected epochs from
the same subject's EEG data,
and another in which shuffled error rates
were compared with EEG epochs selected randomly from all sessions.
The resulting significance planes were nearly indistinguishable,
and the second method was chosen.
Return to text.
5.
Note that this Monte Carlo method of determining the statistical
significance of the coherence amplitude estimates was
unaffected by the fact that two sessions each from three subjects
were used in the computations.
The validity of the Monte Carlo procedure depends only on
how accurately the shuffling employed
simulated the null hypothesis of no linear relationship between
the respective pairs of DFT's, not upon the fact that each pair is
derived from a different individual.
We explore individual subject differences using three other methods
(Figs. 6-8).
Return to text.
6.
Note that simultaneity is measured in terms of fraction of a performance
cycle, i.e. in seconds rather than milliseconds.
Return to text.
7.
The large region of insignificant coherency in the lower right portion of
the coherency planes is also
the region both of lowest EEG power
and of lowest error rate fluctuation amplitude.
Therefore significant coherency in this region
could be obscured by estimation errors introduced by
muscle activity, quantization errors, or finite window length.
Return to text.
8.
To examine the claim of Ogilvie et al. (1991), we computed the
power spectra for the 5 seconds preceding targets presented during the
first minute of periods of no response.
These were then compared with the power spectra derived
from the first two minutes of the corresponding sessions
in which performance was nearly perfect (see Fig. 1).
Our pattern of results did not change, and did not replicate their claims
that EEG power increases in all bands during the one to two minutes following
first errors of omission.
The major difference in most cases was the prominent drop in power in
the alpha band, which has also been reported by several other authors
during eyes-closed sleep transitions.
Return to text.
9.
Maps of multiple correlations computed independently for each site showed
maximum predictive capability at Fz and F4.
A slight right-sided bias in correlation was found at
all four homologous right/left channel pairs,
but was not significant by t-test across the 10 subjects.
Return to text.
Your email comments to first author?
Back to publication list.
Introduction
Methods and Materials
Analysis
Results
Discussion
Relationship of EEG and Alertness
Time Structure
Possible Applications
Conclusions
Acknowledgements
References
Footnotes