CHANGES IN ALERTNESS ARE
A PRINCIPAL COMPONENT OF
VARIANCE IN THE EEG SPECTRUM
NeuroReport 7:213-217 (1995)
Scott Makeig
The Salk Institute
La Jolla CA
smakeig@ucsd.edu
and
Tzyy-Ping Jung
Computational Neuroscience Laboratory
The Salk Institute
La Jolla CA
Download 55k compressed Postscript version of this article.
General Summary
We correlate minute-scale fluctuations in the normalized EEG log spectrum during drowsiness with concurrent changes in level of performance on a sustained auditory detection task, and show that a single principal component of EEG variance is linearly related to minute-scale changes in detection performance. The EEG frequencies at which this coupling is expressed are similar for most subjects during single- or dual-task and eyes-open or eyes-closed conditions. This pattern of performance correlations across EEG frequencies closely matches the profile of EEG frequency changes recently reported from analysis of cued verbal self-reports of thinking and awareness during drowsiness. These EEG changes apparently arise from simultaneous changes in brain mechanisms controlling central arousal and alertness and in the levels of coherent neural activity at several characteristic neural oscillation frequencies. The unidimensional relationship between changes in performance and the EEG spectrum during drowsiness may make possible practical methods of EEG-based real-time alertness estimation.
Abstract
We correlate minute-scale fluctuations in the normalized EEG log spectrum with concurrent changes in level of performance on a sustained auditory detection task, and show that a single principal component of EEG spectral variance is linearly related to minute-scale changes in detection performance. The particular EEG frequencies at which this coupling is expressed are similar for most subjects under a range of task conditions, and match those recently reported from analysis of verbal self-reports during drowsiness. The one-dimensional relationship between detection performance and the EEG spectrum confirms quantitatively the intuitive assumption that minute-scale changes in behavioral alertness during drowsiness are predominantly linked to changes in global brain dynamics along a single dimension of psychophysiological arousal.
Key Words: EEG, electroencephalogram, alertness, vigilance, auditory detection, principal component analysis (PCA), spectrum
Disclaimer This Report was supported by a grant ONR.Reimb.30020(6429) to the Naval Health Research Center from the Office of Naval Research. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U. S. Government. Approved for public release; distribution unlimited.
Introduction
One of the earliest observations of changes in the EEG spectrum correlated with behavior was that at transition to sleep, the EEG spectrum mostly shifts towards lower frequencies[1]. The link between changes in behavioral arousal and the EEG spectrum is strong enough that the appearance of the EEG spectrum has long been used as a direct indicator of arousal level[2]. Here, arousal refers primarily to changes in behavior associated with transitions from slow-wave sleep to waking, and alertness, to the waking end of this continuum. Although careful studies of human EEG records have revealed a range of normal and abnormal transitions between waking and sleeping EEG patterns[3], physiological and behavioral arousal, if considered separately from attention[4], are most often thought of as covarying on a one-dimensional continuum (for example, when we say we feel "half-asleep").
Recently, Makeig and Inlow[5] presented data from half-hour experiments in which subjects listened with eyes closed to sounds embedded in white noise, pressing a response button whenever they heard target sounds consisting of brief increases in noise amplitude. They showed that minute-scale fluctuations in local error rate, a moving-average measure of detection lapse probability, were coherent with minute-scale shifts in the EEG power spectrum in several discrete frequency bands, and that these EEG changes could be used to predict the time course of local error rate using individualized models constructed from EEG and performance data collected in previous task sessions. Changes in the EEG spectrum could be used to accurately estimate the percentage of targets detected, i.e., not only to discriminate between alert and asleep conditions, but also to monitor the time course of performance during drowsy periods. Here we report, first, that a similar relation exists between changes in the EEG spectrum and auditory detection performance during eyes-open, dual-task performance, and second, that spectral changes linearly related to alertness form a single principal component of overall variance in the normalized EEG log spectrum.
Methods and Materials
Stimuli
A Concurrent workstation recorded the EEG and delivered auditory stimuli to the subject in a constant 63 dB white-noise background. Stimuli consisted of targets, 300 ms increases in noise amplitude (rise time 150 ms, fall 110 ms), presented 6 dB above each subject's detection threshold in the noise background. Mean target stimulus onset asynchrony (SOA) was 6 s. Non-target tones at two frequencies (568 Hz and 1098 Hz), were also presented in random order at 72 dB (normal hearing level) with SOAs randomly distributed between 2 s and 4 s. Visual targets consisting of 20 consecutive white squares forming a vertical line were produced by a 386 PC with a VGA color display (13-cm wide by 9-cm high) and presented over a video noise background ("snow") composed of 1-mm grey scale squares. Visual targets appeared at a mean rate of 1 per min, and were not correlated with auditory targets. Visual task data will be reported elsewhere.
Task Conditions
Subjects sat in a comfortable chair, a two-button response box resting on a pillow in their lap, and were asked to press one button whenever they heard a target noise burst, and the other whenever they detected a visual target. Ambient light level was kept low in the small (5'x5') subject chamber. Under these conditions, the white noise background and the monotony of subjects' tasks proved soporific for many subjects who found it difficult to remain alert throughout entire half-hour sessions. From a total of five sessions on each of 15 subjects, pairs of sessions from 10 subjects (9 male/1 female, age range 18-39) contained sufficient lapses in auditory detection (68 +- 32 lapses per session, 24% +- 11% of targets presented) and were used in the analysis.
EEG Recordings
EEG data were collected at two scalp sites, Cz and Pz/Oz (midway between Pz and Oz), referenced to the right mastoid, and from horizontal and vertical bipolar EOG leads, at 12-bit resolution with a sampling rate of 312.5 Hz and a pass band of 0.1-100 Hz.
EEG Spectra
Time-varying amplitude spectra for each channel were computed using 512-point fast-Fourier transforms (FFTs) of 50% overlapping (Hanning-windowed, zero-padded) 256-point data epochs, after rejecting epochs contaminated by eye movements or other artifacts producing excursions of 50 uV or more in any of the EEG or EOG channels. Spectra were then converted to a logarithmic scale and smoothed with a causal 95-s exponential window (90% down 95 s before its leading edge), yielding for each session 1024 smoothed log spectral estimates at 1.6384-s intervals. The log spectrum for each session was then normalized separately for each of 40 frequency bins between 0.61 and 24.4 Hz by subtracting the session mean and dividing by the 25-75 percentile range of the resulting time series.
The normalized log spectra at Cz and Pz/Oz from the 20 sessions on 10 subjects selected for analysis were then submitted to principal component analysis (PCA)[6] producing 80 eigenvectors and 80 corresponding eigenvalues. PCA eigenvectors with largest eigenvalues represented directions of largest multi-dimensional variance in the log spectrum during the experiments.
Detection Performance
Hits and lapses were defined as auditory targets responded to or not responded to within a 100-3000 ms window following target onset. Auditory task error rate was smoothed using the same 95-s moving exponential window used to smooth the EEG spectrum by multiplying a performance index (0 for each hit in the moving window, 1 for each lapse) by the appropriate window weight (determined by the relative time of occurrence of each target within the window), summing the results, then normalizing the result by dividing by the sum of the window weights used in computing the sum. The performance smoothing window was moved through the performance index in 1.6384-s (512-point) steps, converting the irregularly-spaced, discontinuous performance index into a regularly-spaced, continuous local error rate measure representing the current probability that the subject will fail to respond to a presented target.
Correlation Spectra
For each session, correlations between smoothed log spectral and error rate time series were then computed separately for each of 40 FFT frequency bins from 0.6 Hz to 24.4 Hz. Results were said to form the "correlation spectrum" of each experiment. A mean correlation spectrum was computed by averaging correlation spectra from the 20 sessions. Significance levels for the resulting mean correlation values were estimated using Monte Carlo methods. First, for each session the time course of log power at each of 40 frequency bins in the two channels was correlated with the time course of error rate in the other 19 sessions. Next, 500 surrogate mean correlation spectra were computed by averaging pseudo-randomly-selected sets of 20 of these surrogate correlations. For each frequency bin, the 5th, 50th, and 95th percentiles of the resulting correlation distribution were then identified. In addition, a grand correlation spectrum was computed for all 20 sessions simultaneously using the same normalized data used to compute the PCA.
Results
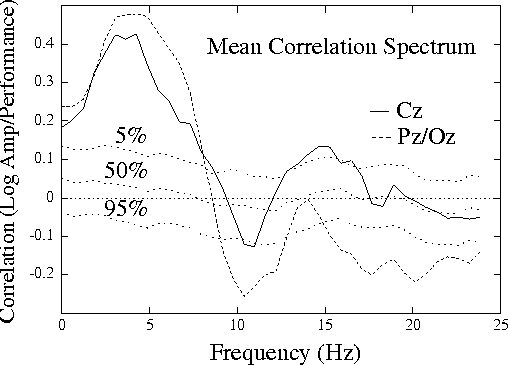
Figure 1a.
Grand mean correlation spectrum,
showing mean correlations between EEG log power
and local error rate (the probability of
an auditory detection lapse)
for 20 half-hour sessions on 10 subjects,
both measures smoothed using a causal 95-sec exponential
window. Correlation spectra at two scalp channels, Cz and Pz/Oz,
are superimposed on dotted traces showing the 5th, 50th, and 95th
percentiles of the distribution of surrogate correlations computed
by correlating EEG power and error-rate time series from separate
sessions.
Mean and grand correlation spectra were nearly identical
(r = 0.995, rms difference = 0.023).
Fig. 1a (above) shows the mean correlation spectrum at the two
sites, superimposed on traces showing significance levels
from the surrogate correlation distribution (see Methods).
Correlations between EEG power and
performance are significant in four frequency bands: (1) at 4-5 Hz
(theta) in both channels, (2) at 10-11 Hz (alpha) at Pz/Oz,
(3) at 14-15 Hz (sigma) at Cz,
and (4) above 15 Hz (beta) at Pz/Oz.
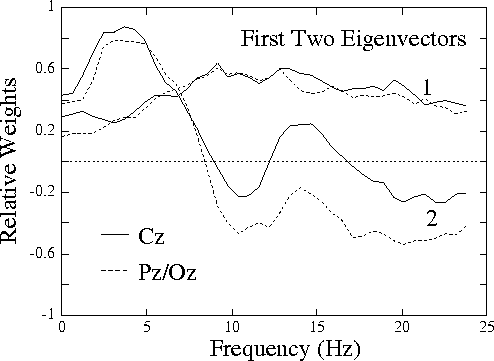
Figure 1b.
Plot of eigenvectors corresponding to the first two
principal components of variance of the normalized EEG log spectrum
in the 20 sessions. While the near-constant eigenvector 1 is
(like eigenvectors 3-80) little-related to performance,
eigenvector 2 is highly related (see Fig. 2).
Fig. 1b (above) shows the eigenvectors corresponding to the two
largest principal components of EEG variance in the sessions. The
first component, accounting for 27% of the total variance, is
uniformly positive (or negative). The second component, accounting
for 16% of variance, strongly resembles the correlation spectrum
shown in
Fig. 1a,
with maxima near 4 Hz (in both channels) and 14
Hz (at Cz only), and minima near 10 Hz and above 20 Hz.
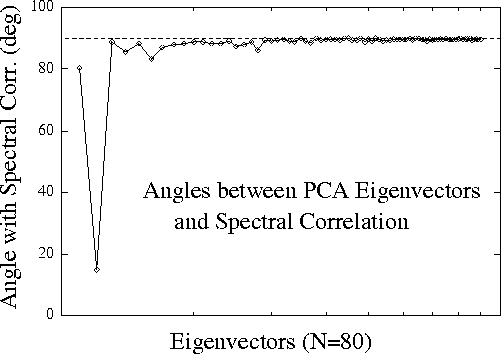
Figure 2.
Vector angles between the 80 PCA eigenvectors characterizing
variance in the normalized EEG log spectrum, and the grand correlation
spectrum vector consisting of correlations between performance and
normalized EEG log power at 40 EEG frequencies and 2 scalp channels
during all 20 sessions. Only the second PCA eigenvector is aligned with
the performance correlation vector; all other principal components
of EEG variance are nearly orthogonal to it.
Fig. 2 (above)
plots the angles between each of the 80 PCA eigenvectors
and the grand correlation spectrum. The second eigenvector is
aligned within 15 degrees of the correlation-spectrum vector, while
all other eigenvectors are nearly orthogonal to it. Accordingly,
the projection of the EEG spectral data on the second eigenvector
correlated nearly as highly (r = 0.58) with the time course of
error rate as multiple regression using projections on the first
eight eigenvectors combined (r = 0.60). This result confirms that
nearly all performance-related changes in the EEG spectrum are
confined to one principal component (or eigenvector) of spectral variance.
First, we now have found nearly identical linear relationships between
normalized EEG log spectra and minute-scale changes in auditory detection
probability during both single-[5] and dual-task experiments.
Except near 10 Hz, the mean correlation
spectrum in the current (dual-task, eyes-open) experiments
(Fig. 1a)
strongly resembles previous results obtained under single-task,
eyes-closed conditions[5], the stronger 10 Hz performance
correlation during eyes-closed conditions reflecting the well-known
decline in closed-eyes alpha during drowsiness[1].
Amplitude changes at theta, alpha, sigma, and beta frequencies
have long been known to be
associated with drowsiness and sleep onset[1]. Our results
quantify the extent to which these changes covary linearly with
performance changes on a continuous detection task.
Further evidence for the generality of the EEG correlation spectrum
shown in
Fig. 1a
appears in a recent paper by Lehmann et al.[7],
who put subjects to bed in a darkened room at their normal bedtimes,
then prompted them, at 7-minute intervals, to say whatever was on
their mind at the moment the prompt sounded. After blind rating
of the recorded verbal responses on 20 bipolar rating scales, the
scaling data and EEG spectra just prior to the prompts were analyzed
using canonical correlation. The largest correlation factor extracted
by this procedure was most heavily weighted on verbal scale values
associated with loss of recall and body awareness, with remoteness
and indirectness -- all qualities compatible with drowsiness, hypnagogy,
and/or loss of sensory awareness.
Although the (bipolar central-parietal) scalp derivations used by
Lehmann et al. differ somewhat from ours, their first EEG factor
closely resembles our correlation spectrum
(Fig. 1a)
and, even more
strongly, the eyes-closed correlation spectra reported by Makeig
and Inlow[5]. Note that Lehmann et al. used quite a
different task than our experiments, and that, moreover, their
subjects' failures to respond to the prompt were excluded from
their data, while lapses in auditory detection were the subject of
our analysis. Yet, both experiments yield very similar frequency-weighted
components relating changes in alertness and the EEG spectrum during
drowsiness.
The significance of the performance correlation peaks in
Fig. 1a
implies that the frequencies at which log power was correlated with
detection performance are similar for most subjects. Examination
of correlation spectra for individual subjects confirmed this
conclusion, although stable between-subject differences also
exist[5,7]. Note also that our finding of a significant
log-linear component in the relation between EEG and performance
does not preclude the possibility that this relationship also
contains other nonlinear terms, including performance floor effects,
EEG saturation, or others. Such factors may make nonlinear fitting
algorithms or artificial neural networks more efficient than
linear regression in estimating changes in alertness from
EEG records[8].
Second, we have found that the mean correlation spectrum for these
sessions nearly parallels a single principal component of
variance in the normalized EEG log spectrum during the sessions,
and is nearly orthogonal to all others
(Fig. 2).
Performance-related variations in this component of EEG spectral
variance during these experiments are apparently produced by changes
in brain arousal which are closely linked to changes
in the probability of detecting (and/or responding to)
above-threshold signals. These changes in detection probability
may be caused by intermittent or noisy gating of the delivery
of efferent auditory information
to the cortex[9], or possibly by drowsiness-related changes
in other brain subsystems involved in performing the task.
In our experiments, performance decrements appear as waves
of (usually) intermittent detection lapses
lasting four minutes or more[5] and containing
characteristic 15-20 s cycles[10].
The concentration of performance-related EEG spectral changes
in a single eigenvector
suggests a tight coordination of dynamic brain changes
underlying minute-scale changes in arousal and sensory gating
during drowsiness. During drowsiness as seen here
under monotonous task conditions, brain and behavioral arousal do
indeed covary on a one-dimensional continuum, as often assumed. It
should be interesting to test whether similar results are obtained
when links between EEG changes and behavioral alertness are examined
using more complex or demanding tasks.
As has long been known, changes in brain arousal involve specific
changes in oscillatory brain activity[1-3,5,7-10].
Our results show that correlations between minute-scale changes
in the EEG log spectrum and performance on a sustained auditory detection task
are similar for most subjects.
At the two central scalp sites we have studied and except near 10 Hz,
correlations between performance
and EEG log amplitude are similar in eyes-open
and eyes-closed conditions involving dual- or single-modality[5]
detection tasks, and closely match those recently reported based on
subjects' self-reports near sleep transitions[7].
During the current dual-task, eyes-open experiments, minute-scale changes
in the frequency of auditory detection lapses were predominantly
correlated with changes in the normalized EEG log power spectrum
along a single principal component or eigenvector of EEG spectral variance.
These EEG changes apparently index coordinated changes
in activity of brain systems controlling central arousal
(and/or auditory gating) which result in behavioral changes
along a one-dimensional drowsy-alert continuum, as commonly assumed.
Elsewhere, we have shown that these EEG spectral correlates of loss
of alertness can be used to monitor the timecourse of alertness
in near real time, for scientific or applied purposes[8].
Acknowledgements
We acknowledge the contributions of F. Scot Elliott and Mark Postal
in collecting and processing the data, and the technical support
of Terrence Sejnowski of The Salk Institute, La Jolla. The
project was supported under work unit ONR.Reimb.30020(6429)
by the Office of Naval Research.
1. Davis H, Davis P, Loomis A, et al. J Neurophysiol 1,
24-38 (1938).
2. Steriade M, McCarley RW. Brainstem Control of Wakefulness
and Sleep. New York: Plenum, 1990, 498 pp.
3. Lehmann D, Grass P, Meier B. Int J Psychophysiol 19,
45-52 (1995).
4. Pribram KH, McGuinness D. Ann. New York Acad. Sci.
658,65-92 (1992).
5. Makeig S, Inlow M. Electroencephalogr clin Neurophysiol,
86, 23-35 (1993).
6. Sneath PH, Sokal RR, Numerical Taxonomy: the Principles and
Practice of Numerical Classification, San Francisco: WH Freeman,
1973.
7. Santamaria J, Chiappa KH. J. Clin. Neurophysiol., 4,
327-382 (1987).
8. Jung T-P, Makeig S, Stensmo M, and Sejnowski TJ, IEEE
Trans. Biomed. Eng., (in press).
9. Steriade M, Dossi RC, Pare D. Mesopontine cholinergic systems
suppress slow rhythms and induce fast oscillations in thalamocortical
circuits. In: E Basar, TH Bullock, eds. Induced Rhythms in the
Brain. Amsterdam: Elsevier, 1988: 251-268.
10. Makeig S, Jung T-P, Cogn. Brain Res. (in press).
Discussion
Conclusion
References